
Referral Tips
Start communication with patients and CAR T treatment centers before disease recurrence1-3
Referral tips to expedite the CAR T process

Proactively identify potential CAR T patients
- Request consultation for your patients at first suspicion of relapse3
- Prior to initial consultation, consider preparing and compiling patient medical records to provide to the CAR T treatment center1,4
- This can include patient medical history, recent diagnostic scans, performance status, and pathology results
- Upon referral, immediate insurance verification can occur to assess coverage4

Get to know the members of the multidisciplinary CAR T care team
- Key treatment center team members include the CAR T treater, advanced practitioners, and nurse coordinators1
- It may be helpful to provide direct contact information to ensure timely communication. The care team may need to work with your team on determining patient eligibility for treatment, timeline for therapy, and any plans for chemotherapy prior to CAR T-cell therapy infusion
- Treatment coordinators are helpful resources who can offer support throughout the entire referral process, including financial, transportation, and housing assistance5

Work closely and directly with CAR T treaters
- Patients may have a better chance of receiving treatment if there is early and effective communication between your office and CAR T treaters1
- Informing the CAR T treatment center of a potential patient in advance allows the center to ensure spots are available and helps them to prioritize patients
- You may want to consider referring your patients directly to CAR T treaters so they can discuss CAR T in further detail

Consultations can be made easier by:
- Having the cellphone number of a verified CAR T treater1
- Considering telemedicine appointments6
- Utilizing EHR systems6

Organizations, such as The Leukemia & Lymphoma Society,* are valuable resources that are eager to become an extension of your clinical team
- They can also help navigate referral, financial, and logistical issues (www.lls.org)
*These resources are not operated or controlled by Kite. Eligibility requirements may vary and are established solely by each independent organization.
Patient consultation for CAR T therapy should occur upon treatment failure to help streamline the referral process and reduce wait times for treatment initiation.1-3
CAR T Patient Journey
Collaboration and communication are key throughout the CAR T journey
Close collaboration between the primary oncologist, CAR T treater, and patient provides continuous insight and informed treatment decisions regarding overall continuity of patient care.











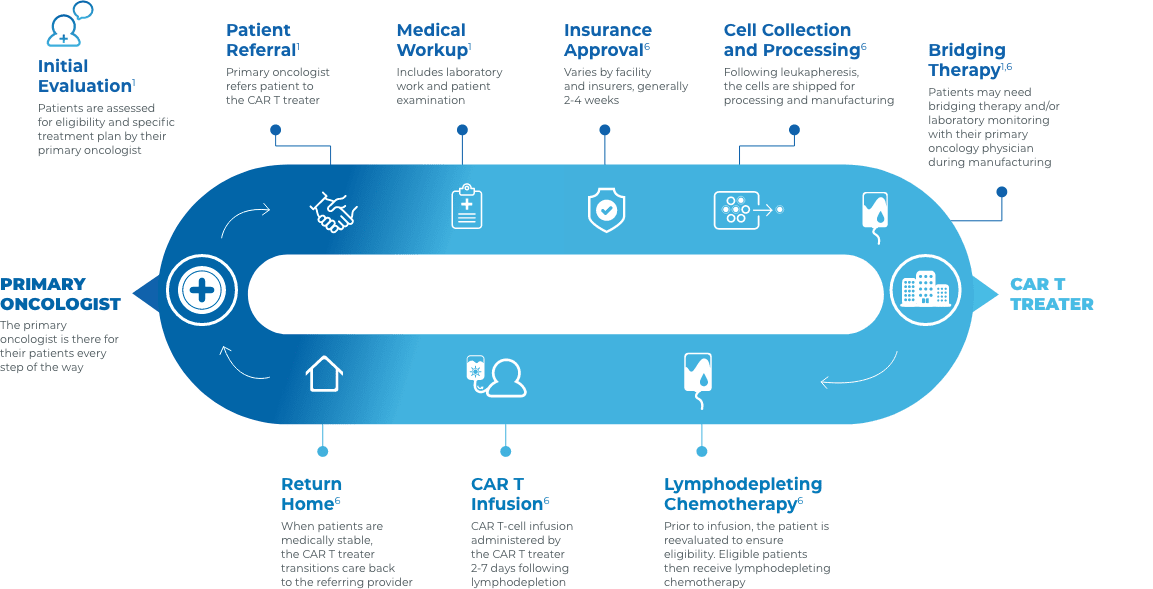
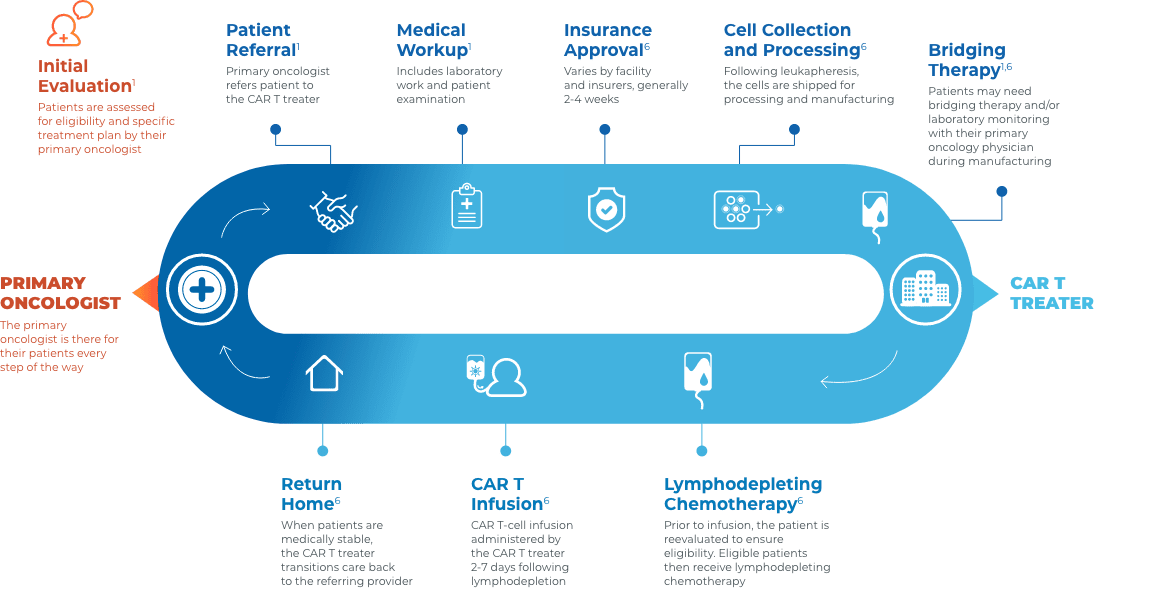
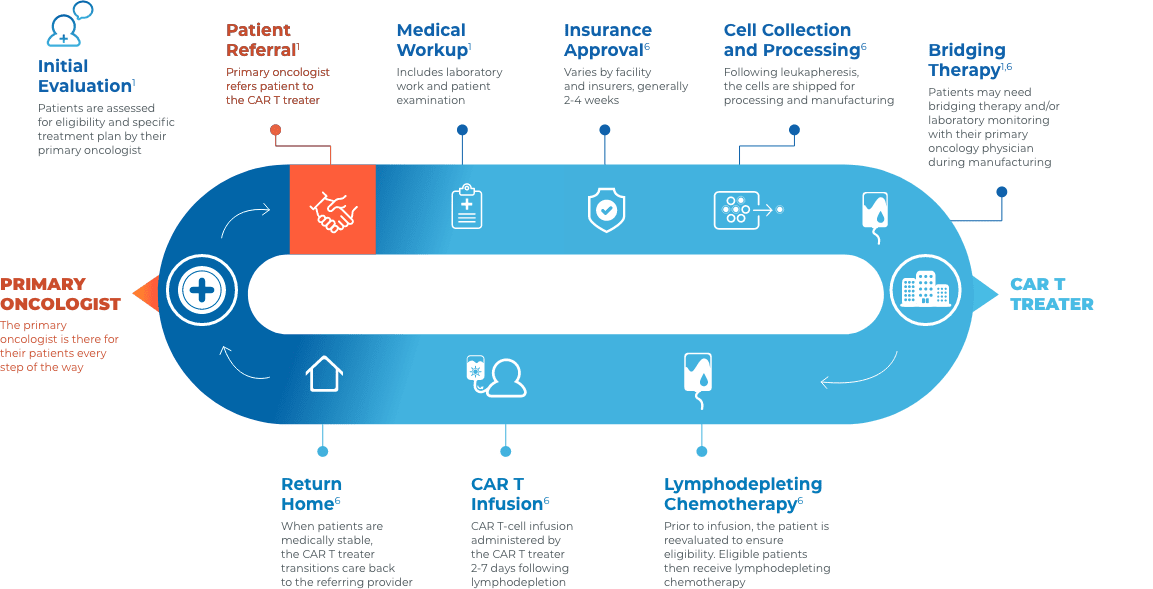
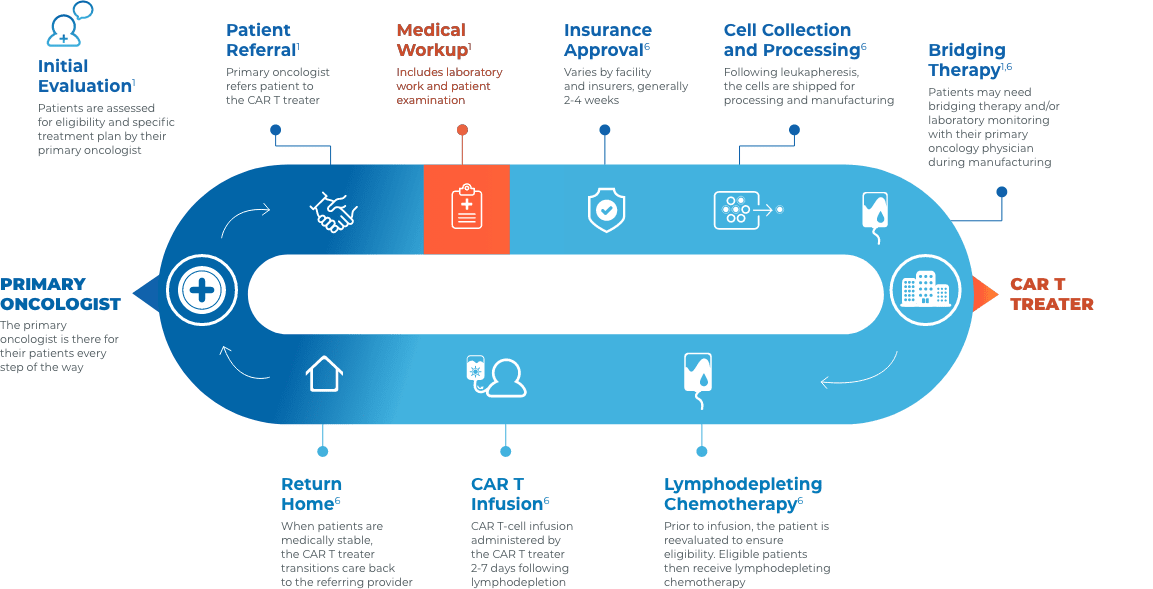
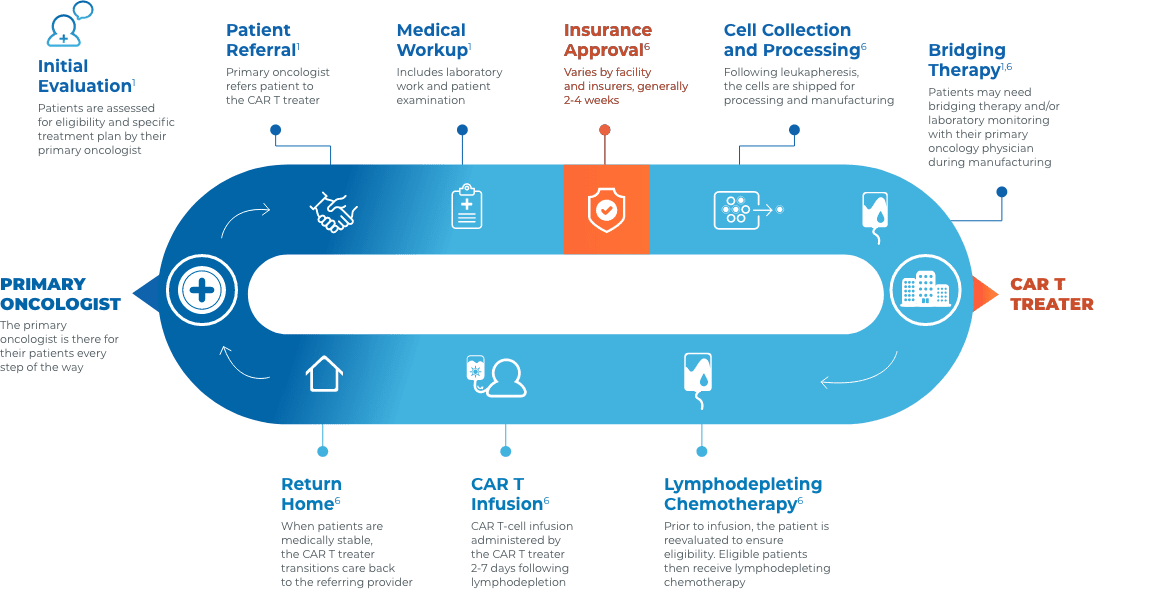
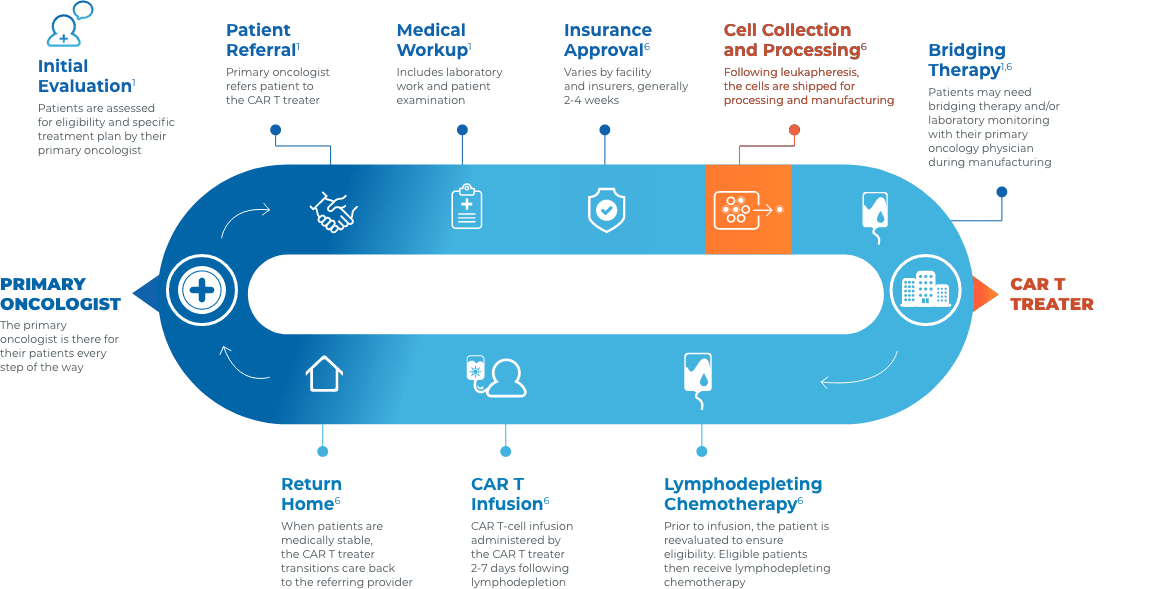
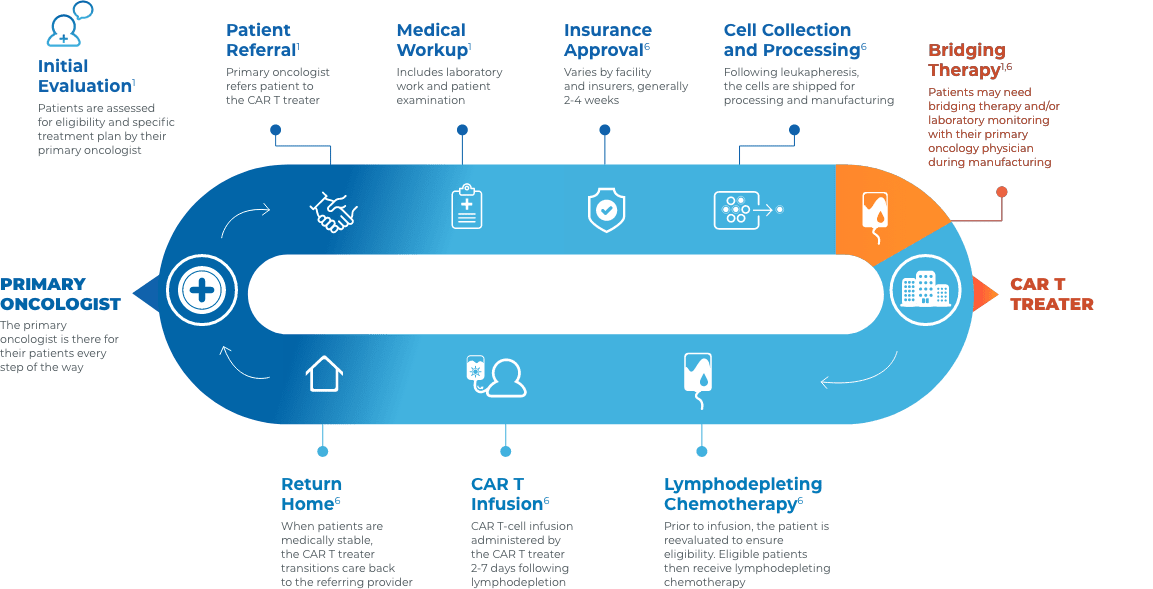
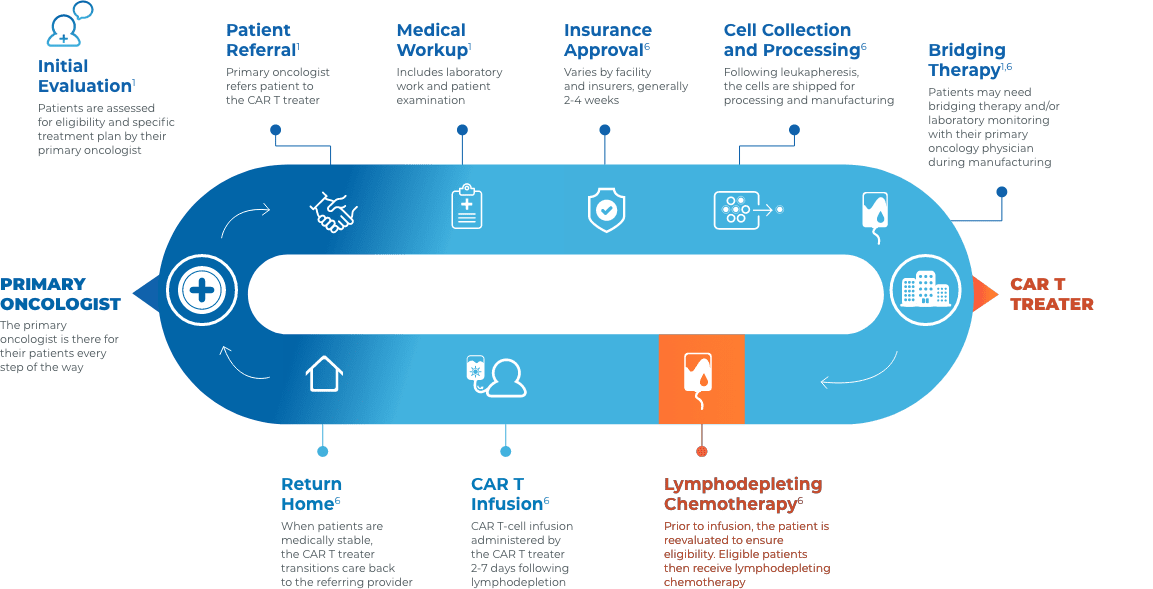
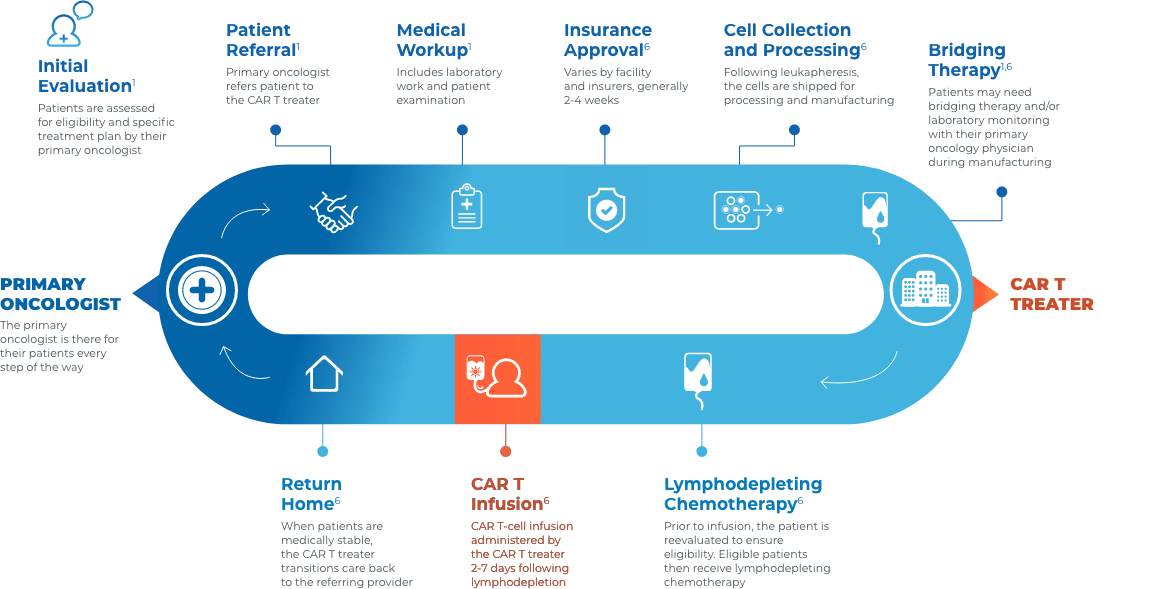
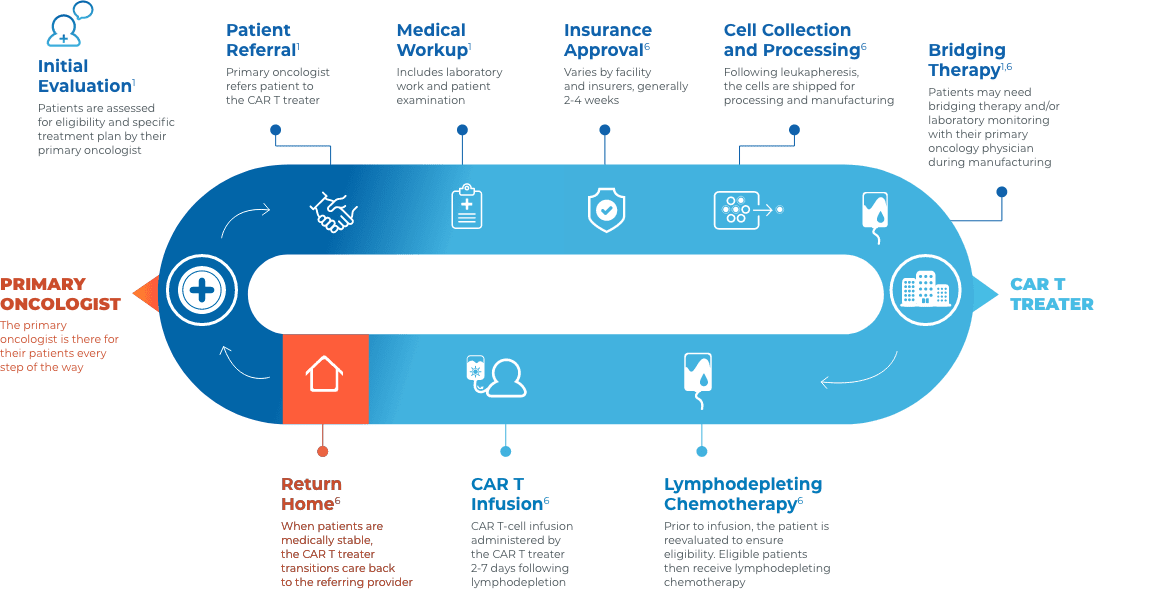
Stay connected with your patient’s care

Before treatment
- Educate patients on CAR T as an option in the event of relapse or refractory disease3
- Help patients understand the breadth of clinical experience and adverse event management expertise of their multidisciplinary care team3
- Encourage patients to visit LetsChatCART.com—a patient website that provides educational information on how CAR T works, what to expect when receiving CAR T, resources for caregivers, and where to find additional support

During treatment
- Patients will be at, or near, the CAR T treatment center for approximately 28 days for treatment and monitoring1
- Most acute AEs occur within this time frame and are managed at the CAR T treatment center. CRS and neurologic toxicities post 28 days are rare1,7,8

After treatment
- Primary oncologists typically do not need to manage acute adverse events1,7,8
- To ensure a smooth transition of care back to your practice, consider requesting some or all of the following1,6:
- A copy of the CAR T therapy prescribing information, medication guide, and patient wallet card
- An accurate and up-to-date medication list
- Hospital records (eg, pre-CAR T therapy workup results, physician notes during inpatient treatment and from the last ambulatory visit, restaging results if performed on days 28-30)
- Information about CRS and neurologic events
- Recommendations of appropriate laboratory values to monitor, as well as the testing frequency
- Monitor for late effects (ie, cytopenias, hypogammaglobulinemia, and secondary malignancies)1
- Contact the CAR T treater immediately if there is a relapse post therapy3

AE=adverse event; CAR=chimeric antigen receptor; CAR T=chimeric antigen receptor T cell; CRS=cytokine release syndrome; EHR=electronic health record.
References











