
Manufacturing
The CAR T manufacturing process continues to be enhanced and standardized1
High manufacturing success rates of 91% to 100% were seen in clinical trials2-10
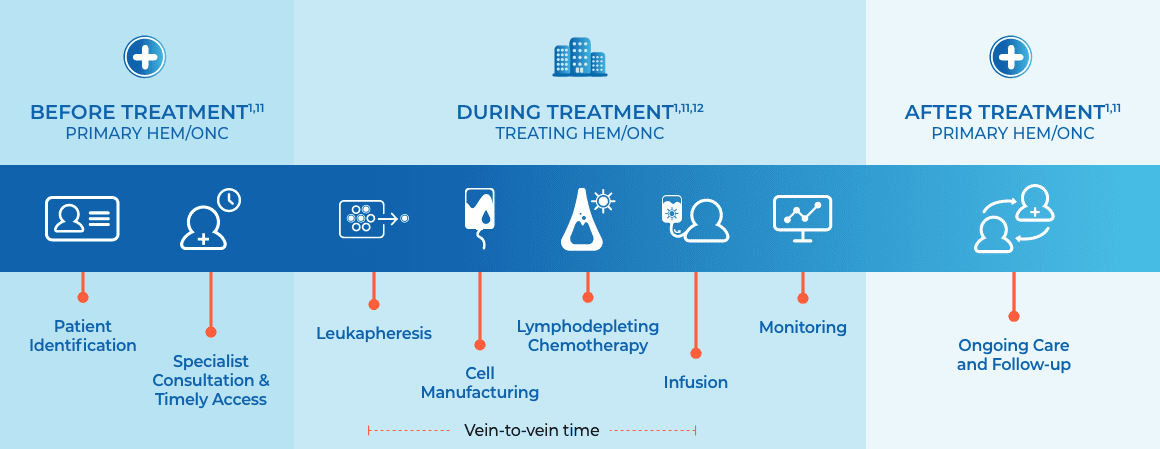
- CAR T-cell manufacturing involves leukapheresis, processing, manufacturing of cells, and shipping1
- “Vein-to-vein” time refers to the time between leukapheresis and infusion1
- Turnaround time refers to the time from leukapheresis to when the CAR T cells are released13
- Bridging therapy can also be introduced, which can include chemotherapy, corticosteroids, or other therapies deemed appropriate to stabilize the patient during CAR T-cell manufacturing1,13,14
Once a patient is identified as eligible to receive
CAR T, the treatment process can be completed in as little as 3 weeks.1,11
Kite manufacturing is committed to predictable, reliable, and flexible delivery
Although turnaround times and product specifications vary across CAR T therapies, manufacturers strive to achieve fast turnaround times, in-spec target doses, and convenient apheresis and delivery dates to ensure timely administration.
CAR T Resources
You support your patients. Support is here for you.
Keep the CAR T Hope Brochure with you to share and highlight key information about CAR T therapy with stakeholders in your practice.
Learn what you need to know about CAR T
Download CAR T Hope Brochure
Looking for a treatment center?
Timing matters. Find the closest treatment center to refer your patients today.
*Based on US commercial manufacturing data as of October 2022.15
†Based on commercial manufacturing data as of October 2022.16
CAR=chimeric antigen receptor; CAR T=chimeric antigen receptor T cell.
References
1. Geethakumari PR, Ramasamy DP, Dholaria B, Berdeja J, Kansagra A. Balancing quality, cost, and access during delivery of newer cellular and immunotherapy treatments. Curr Hematol Malig Rep. 2021;16(4):345-356. 2. Kamdar M, Solomon SR, Arnason J, et al; TRANSFORM investigators. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294-2308. 3. Sehgal A, Hoda D, Riedell PA, et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. Lancet Oncol. 2022;23(8):1066-1077. 4. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. 5. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. 6. Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705-716. 7. Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314-324. 8. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. 9. Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol.
2022;23(1):91-103. 10. Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654. 11. Berdeja JG. Practical aspects of building a new immunotherapy program: the future of cell therapy. Hematology Am Soc Hematol Educ Program. 2020;2020(1):579-584. 12. Gajra A, Zalenski A, Sannareddy A, Jeune-Smith Y, Kapinos K, Kansagra A. Barriers to chimeric antigen receptor T-cell (CAR-T) therapies in clinical practice. Pharmaceut Med. 2022;36(3):163-171. 13. Beaupierre A, Kahle N, Lundberg R, Patterson A. Educating multidisciplinary care teams, patients, and caregivers on CAR T-cell therapy. J Adv Pract Oncol. 2019;10(suppl 3):29-40. 14. Amini L, Silbert SK, Maude SL, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol. 2022;19(5):342-355. 15. Data on file [1]. Kite Pharma, Inc; 2022. 16. Data on file [2]. Kite Pharma, Inc; 2022. 17. Data on file [3]. Kite Pharma, Inc; 2022.


