
PATIENT IDENTIFICATION
Patient identification for CAR T is largely dependent on primary oncologists
- Over 50% of all US patients battling cancer are treated in community practices1
- Perceived barriers for CAR T use include2-4:

Older patient age

Safety concerns

Comorbidities

Administrative/logistical challenges
- A majority of oncologists reported that 50% or less of their patients referred for CAR T treatment received it5*
- Patients may progress before receiving CAR T and become ineligible for treatment. Often patients have moved onto subsequent lines of therapy and their disease may progress rapidly, underscoring their immediate need for treatment2,6
You have an essential role throughout the CAR T journey
Find out who is eligible for CAR TImprove the rate of successful referral through early identification of appropriate patients for CAR T
Who is eligible for CAR T therapy?

Current criteria for receiving CAR T are less strict than those of the pivotal trials.4,7,8
- As many as 56% of patients observed in real-world studies would not have been able to participate in clinical trials due to comorbidities7-11
- Many appropriate patients for CAR T, who were ineligible for clinical trials, are now being referred to treatment centers4

Patients should be selected based on their medical history and physical condition.4,12
Other factors to consider when referring a patient for CAR T include†:
- Age
- ECOG performance status
- Organ function
- Bone marrow reserve
- History of active malignancy
- Presence of CNS involvement
- Existing or suspected infection
- Major cardiac conditions
- Pulmonary function
- Prior transplant

Patients with aggressive R/R B-cell NHLs are historically associated with a poor prognosis and should be evaluated promptly for CAR T eligibility.13,14

Evaluating patients with hematologic cancers for CAR T eligibility upon treatment failure may help accelerate the time to treatment initiation.6,14,15
More of your patients may be eligible to receive CAR T therapy than you think. Consult with a CAR T treater upon treatment failure for timely assessment.
CAR T Experience
22,000+ patients with certain hematologic cancers have been treated with CAR T therapy globally16,17
A broader range of patients than those studied in clinical trials may benefit from CAR T therapy7,8,15,18,19
- Real-world evidence was generally consistent with the efficacy and safety results seen in clinical trials7,8,15,18,19
- Some patients who received CAR T in real-world studies had an ECOG performance status ≥220
-
Stem cell transplant has general age guidelines (<65 years of age), while CAR T does not, enabling greater eligibility for patients to receive CAR T therapy4,21
- Patients who received CAR T in real-world studies ranged from 14 to 91 years of age7,9,18,22,23
Outcomes in patients ≥65 years and <65 years of age from clinical trial and real-world evidence24-26‡
| ORR | CR | |||
|---|---|---|---|---|
| ≥65 years | <65 years | ≥65 years | <65 years | |
| LBCL | 59-92% | 49-81% | 51-75% | 48-53% |
| FL§ | 90% | 94% | 79% | 71% |
| ALL | NA | 100% | 71% | |
| MM|| | 70%-97% | 90% | NA | |
| ORR | CR | |||
|---|---|---|---|---|
| ≥65 years | <65 years | ≥65 years | <65 years | |
| LBCL | 59-92% | 49-81% | 51-75% | 48-53% |
| FL§ | 90% | 94% | 79% | 71% |
| ALL | NA | 100% | 71% | |
| MM|| | 70%-97% | 90% | NA | |
Common adverse events associated with CAR T therapy are CRS and neurotoxicity. Some side effects can be severe. Please refer to the safety section for additional information.14
Impact of Wait Times
Start the CAR T referral process once you have identified a potential patient6,27
Wait time management is an important factor to consider, particularly in patients with hematologic malignancies who have a poor prognosis. Promptly refer eligible patients to CAR T treatment centers to avoid potential treatment delays and other unfavorable outcomes.6,13,14,27
Wait times can impact patient mortality28
A discrete event simulation model was developed to project the potential impact of wait times on CAR T therapy for patients with R/R DLBCL.
- It simulated a patient population from a clinical trial who had received chemotherapy and assumed that they followed established OS and PFS chemotherapy curves
- 9 hypothetical wait times (1 to 9 months) in addition to a 2-3 week CAR T manufacturing time were used
- The outcome was all-cause mortality set to 1 year
-
Limitations include the single-arm nature of trials used and the lack of randomized trials for parallel two-arm comparison between CAR T therapy and chemotherapy
- In this analysis, it was assumed patients would immediately receive chemotherapy during the delay of treatment, therefore the impact on wait times could be underestimated or overestimated
- The model design affects the generalizability of these findings and should not be used to guide individual patient decisions
Increase in all-cause 1-year mortality rate relative to the baseline 1-year mortality rate of 34.0%28
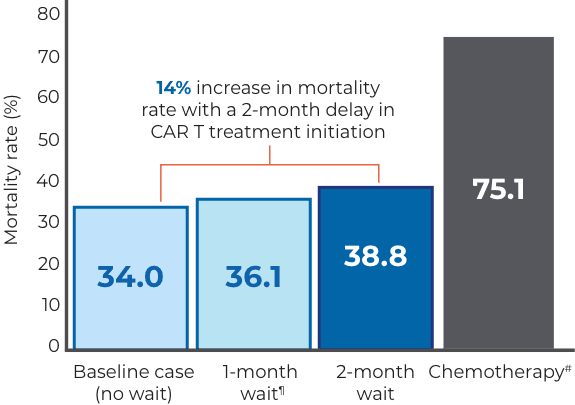
Prolonging the wait time for CAR T therapy from 1 to 9 months increased the predicted 1-year mortality rate from 36.1% to 76.3%.28
The magnitude of survival decreases as wait time for treatment increases28
Survival rates could decrease as the wait times for CAR T therapy increase.28

*In preceding 6 months. Based on a 2021 Cardinal Health survey of 154 oncologists.5
†This is not a comprehensive or definitive list of eligibility criteria for CAR T referral. Patient selection should be based on the individual and can vary among CAR T centers.
‡Based on a review of 9 clinical and real-world studies of approved CAR T products in patients with DLBCL, MCL, MM, FL, and ALL.24
§Based on available subgroup data from a single-arm, phase 2 clinical trial that evaluated ORR and CR (n=104) in adult patients with R/R indolent NHL.25,29
||Includes subgroup data from a single-arm, phase 1b/2 clinical trial that evaluated ORR in 35 patients aged ≥65 years with R/R MM.26
¶6.2% increase in all-cause 1-year mortality rate from baseline.28
#121% increase in all-cause 1-year mortality rate from baseline.28
ALL=acute lymphoblastic lymphoma; CAR T=chimeric antigen receptor T cell; CNS=central nervous system; CR=complete remission; CRS=cytokine release syndrome; DLBCL=diffuse large B-cell lymphoma; ECOG=Eastern Cooperative Oncology Group; FL=follicular lymphoma; HCP=healthcare provider; LBCL=large B-cell lymphoma; MCL=mantle cell lymphoma; MM=multiple myeloma; NA=not available; NHL=non-Hodgkin lymphoma; ORR=overall response rate; PFS=progression-free survival; R/R=relapsed/refractory.
References

